FDA Greenlights Breakthroughs to Empower Women’s Health
The year 2025 marked a pivotal shift in women’s healthcare. The FDA granted several key approvals that are changing clinical practice. These advances focus on expanding access, improving diagnostics, and offering new treatments. They address long-standing gaps in care across a woman’s reproductive lifespan.
From new antibiotics to at-home testing, these innovations reflect a clear trend. Healthcare is becoming more personalized, accessible, and preventive. Here are the most impactful FDA authorizations from 2025.
New Treatments for Infections and Menopause
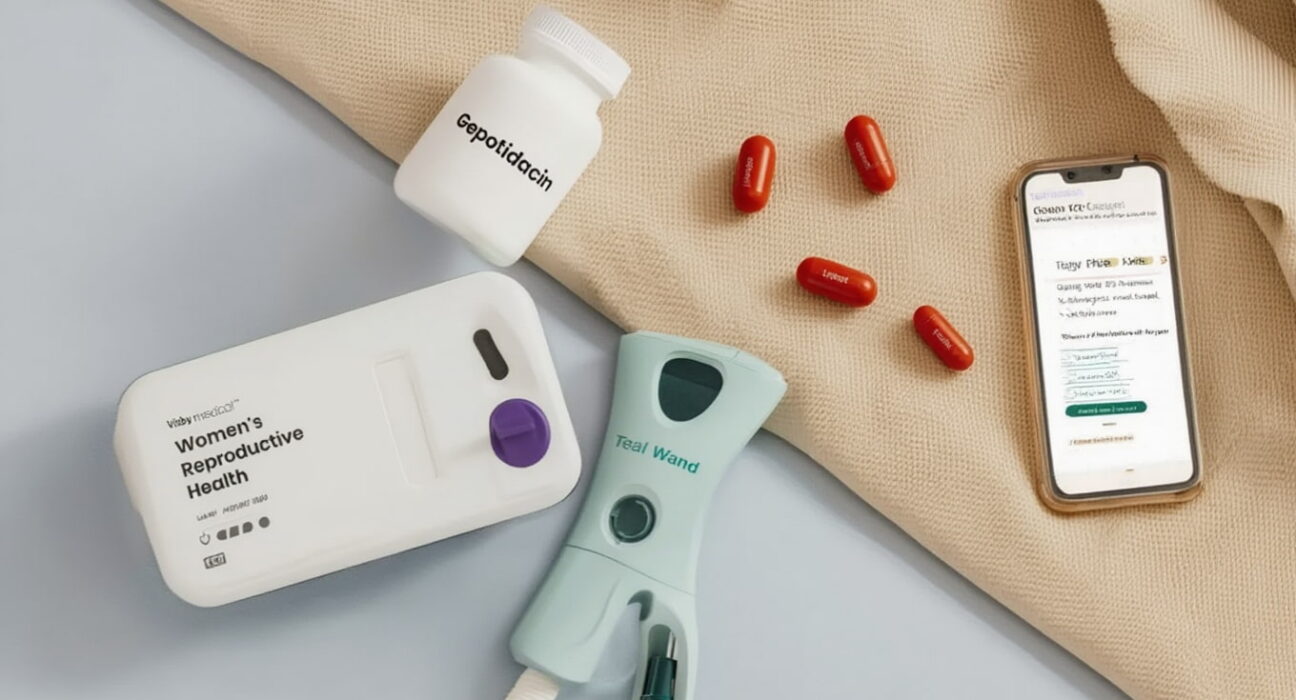
A major focus was on fighting antimicrobial resistance. Gepotidacin (Blujepa) received two important approvals. It is the first new oral antibiotic class for uncomplicated UTIs in over 20 years. Furthermore, it provides a new oral option for treating uncomplicated gonorrhea.
Another oral antibiotic, zoliflodacin (Nuzolvence), also gained approval for gonorrhea. This offers a convenient, single-dose alternative to injections. For menopause, elinzanetant (Lynkuet) was approved. It is the first nonhormonal, dual-action pill specifically for moderate to severe hot flashes.
Advances in At-Home Screening and Diagnostics
Making healthcare more convenient was another key theme. The Visby Medical Women’s Sexual Health Test made history. It is the first prescription-free, at-home test for chlamydia, gonorrhea, and trichomoniasis. Results are available in about 30 minutes via a smartphone app.
Similarly, the Teal Wand gained FDA approval. It is the first at-home vaginal self-collection device for cervical cancer screening. It demonstrated 96% accuracy in trials. This device aims to increase screening rates, especially among underserved populations.
Expanding Options in Reproductive and Sexual Health
The FDA also approved a generic version of mifepristone. This action helps broaden access to medication for pregnancy termination. In sexual health, the indication for flibanserin (Addyi) was expanded. It is now the first nonhormonal drug approved for low sexual desire in both premenopausal and postmenopausal women.
Other notable clearances included AI tools and medical devices. Sonio Suspect AI assists in detecting fetal anomalies during ultrasound. The Revi System offers a new, implantable option for managing urgency urinary incontinence. Together, these 2025 approvals represent significant progress toward more comprehensive and accessible women’s healthcare.