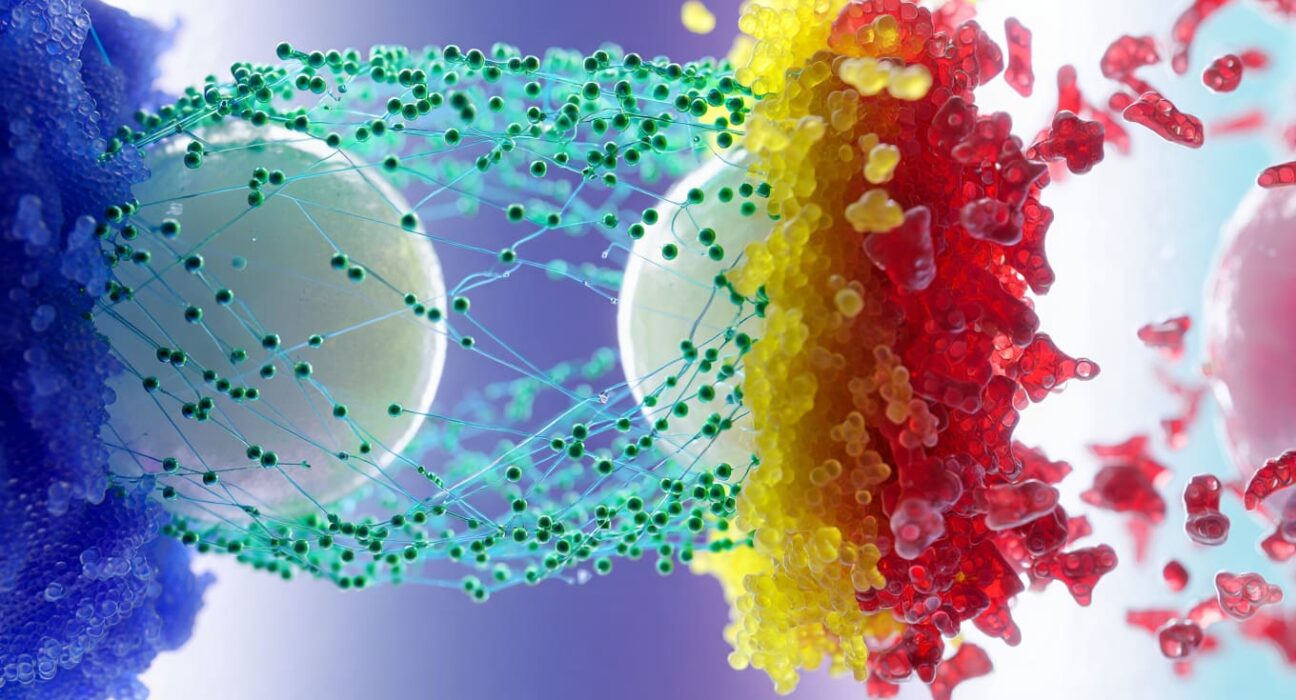
China, have uncovered the explanation. Their findings, published in Nature, reveal a fundamental property of how small molecules interact with larger particles in solution.Traditionally, scientists assumed amino acids stabilized proteins by preventing misfolding during their constant structural fluctuations in solution. However, the team’s research shows that stabilization arises instead from a general colloidal effect, independent of biology.Francesco Stellacci, head of the EPFL lab, compares the phenomenon to two colleagues meeting in a hallway. If the hallway is empty, they easily spot and engage with each other. But in a crowded hallway, they might miss each other or pass by without interaction. Amino acids act like the “crowd,” screening attractive forces between proteins and limiting unwanted interactions.Interestingly, salts are known to have the opposite role: they screen repulsion. Using the same analogy, salts prevent two unfriendly colleagues from avoiding each other, forcing awkward encounters. This makes amino acids essentially an “anti-salt.” In fact, plants watered with salty water produce more amino acids to protect and stabilize their cells under stress.The researchers argue that amino acid concentrations should be reported in scientific experiments with the same rigor as salt levels, since both significantly influence molecular behavior. Looking ahead, Stellacci’s team plans to harness this discovery to predict which small molecules can best stabilize specific proteins—shifting biomedical research away from trial-and-error toward more precise design.
Scientists uncover how amino acids stabilize proteins in solution